A Timing Tangle: Which Common Drugs Require You to Space Out Your Zinc Supplement?
The Unseen Choreography: Navigating the Body’s Intricate Ballets
In the grand, intricate theatre of human physiology, countless biochemical ballets unfold every second. Nutrients, enzymes, hormones, and cellular messengers dance in a complex, often unseen choreography, each movement precise, each timing critical. Among these unsung heroes, zinc stands out – a veritable maestro orchestrating over 300 enzymatic reactions, a silent guardian of immune function, a vital component in DNA synthesis, wound healing, and even the nuances of taste and smell. It is a miniature marvel, essential for life itself.
Yet, even a maestro can be disrupted. What happens when the spotlight shifts, when a new player enters the stage with its own script, its own timing? This is the essence of the "timing tangle" we aim to unravel: the complex, often overlooked interactions between common prescription medications and the seemingly innocuous act of taking a zinc supplement. For the knowledgeable individual, understanding these interactions isn’t just an academic exercise; it’s a critical component of informed self-care, a vital step in ensuring both the efficacy of their medications and the continued support of their nutritional well-being.
The stakes are higher than one might initially imagine. An interaction might diminish the absorption of zinc, leading to subclinical deficiencies that erode health over time. Conversely, zinc might interfere with the absorption or action of a life-saving drug, inadvertently undermining its therapeutic intent. It’s a delicate balance, a tightrope walk between benefit and unintended consequence. This article is an exploration, a deep dive into the specific drug classes and mechanisms that create this timing tangle, offering clarity and practical guidance for those who seek to master the maze of modern health management. We will delve into the cellular pathways, the chemical bonds, and the physiological nuances that dictate these interactions, illuminating the ‘why’ behind the ‘what’ and empowering you to make choices that optimize your health outcomes. Prepare to become a detective of molecular interactions, uncovering the hidden truths that govern the symphony of your body.
Zinc: The Unsung Hero – A Miniature Marvel with Monumental Roles
Before we dissect the intricacies of its interactions, let us first acknowledge the profound significance of zinc. Often overshadowed by flashier vitamins or more widely publicized minerals, zinc is, in truth, an indispensable trace element, a biological linchpin without which life as we know it would falter. Its ubiquity across biological systems is astounding, reflecting its foundational importance.
Consider its role in the immune system: zinc is crucial for the development and function of immune cells, including T-lymphocytes and natural killer cells. A deficiency can dramatically impair immune response, leaving the body vulnerable to infections. For wound healing, it’s a non-negotiable component, participating in cell proliferation, membrane stabilization, and tissue repair. In the realm of cellular machinery, zinc acts as a co-factor for over 300 enzymes, driving metabolic processes ranging from carbohydrate and protein metabolism to the synthesis of DNA and RNA. It’s integral to cell division, cell growth, and the structural integrity of proteins.
Beyond these fundamental roles, zinc plays a part in maintaining healthy vision, supports proper thyroid function, and is a key player in male reproductive health. It even influences our senses, with deficiencies often manifesting as impaired taste and smell. Given this vast portfolio of responsibilities, it’s no wonder that zinc supplementation is popular, particularly among those seeking to bolster immunity, support skin health, or address specific deficiencies identified through dietary analysis or medical testing.
However, the body’s management of zinc is a tightly regulated affair. Absorption primarily occurs in the small intestine, a process influenced by numerous dietary factors and the body’s current zinc status. Once absorbed, it is transported and utilized throughout the body, with excess excreted. This delicate balance, this finely tuned homeostasis, is what makes zinc so susceptible to disruption when external factors, such as pharmaceutical agents, enter the equation. The stage is now set to examine how this miniature marvel can become entangled in a timing conflict.
The Mechanics of Interaction: When Pathways Collide
To truly understand the "timing tangle," we must first grasp the fundamental mechanisms by which drugs and nutrients interact. It’s not magic, but rather a series of predictable biochemical and physiological events, each governed by principles of chemistry and biology. When it comes to zinc and medications, these interactions primarily occur at several critical junctures within the body.
1. The Intestinal Gatekeepers: Absorption Interference
The most common point of interaction is in the gastrointestinal tract, specifically the small intestine, where zinc is absorbed. Imagine the intestinal lining as a series of selective gatekeepers, specialized transporters designed to usher essential nutrients from the gut lumen into the bloodstream. Many drugs, particularly those that are positively charged or have specific binding affinities, can interfere with this process.
- Chelation: The Primary Villain: This is perhaps the most significant mechanism. Chelation involves the formation of a stable, often insoluble, complex between a metal ion (like zinc) and a ligand (often a drug molecule). This complex, once formed, is too large or too poorly soluble to be absorbed through the intestinal wall. The result? Both the zinc and the chelating drug are often prevented from entering the bloodstream, leading to reduced bioavailability of both. It’s like two dancers getting tangled in a knot, preventing them from moving through the stage door.
- Competition for Transporters: While zinc has specific transporters (e.g., ZIP4, ZnT1), other metal ions can sometimes compete for the same or similar absorption pathways. High doses of certain minerals, particularly iron, can vie for these limited "seats" on the transport system, effectively reducing zinc uptake.
- Altered pH: The Stomach’s Influence: The acidity of the stomach (pH) plays a crucial role in the solubility and ionization state of many minerals and drugs. Zinc, in particular, requires a certain acidic environment for optimal dissolution and subsequent absorption in the small intestine. Drugs that drastically alter gastric pH, such as proton pump inhibitors, can inadvertently create an environment where zinc is less available for absorption.
2. Distribution and Metabolism: The Body’s Internal Highways
Once absorbed, nutrients and drugs travel through the bloodstream and are distributed to various tissues and organs. While less common for zinc, some drugs can theoretically alter the binding of zinc to plasma proteins, or interfere with its cellular uptake or utilization. Similarly, some drugs might alter the activity of zinc-dependent enzymes, though this is a more indirect interaction.
3. Excretion: The Kidneys’ Influence
The kidneys are the body’s primary filters, responsible for removing waste products and excess substances, including minerals, from the bloodstream. Certain medications, particularly diuretics, can influence the renal excretion of zinc. By altering kidney function or the reabsorption processes in the renal tubules, these drugs can lead to an increased loss of zinc in the urine, potentially contributing to deficiency over time, even if absorption is normal.
Understanding these fundamental mechanisms provides the necessary framework for navigating the specific drug-zinc interactions we are about to explore. It’s a testament to the intricate nature of human biology, where a seemingly simple act like taking a pill can ripple through the entire system, necessitating a mindful approach to supplementation.
Navigating the Labyrinth: Specific Drug Classes and Their Zinc Entanglements
Now, armed with an understanding of the mechanisms, let us embark on a journey through the labyrinth of specific drug classes that create a timing tangle with zinc. Each chapter reveals a different facet of this complex interplay, offering insights into why and how these interactions occur, and most importantly, how to mitigate them.
Chapter 1: The Antibiotic Enigma – Foiling Infection, Forgetting Zinc
Antibiotics are among the most prescribed medications globally, powerful tools in the fight against bacterial infections. Yet, their very efficacy can sometimes come at an unexpected cost, creating a significant timing tangle with essential minerals like zinc. The primary mechanism here is chelation, a molecular embrace that renders both participants less available.
Tetracyclines (e.g., Doxycycline, Minocycline, Tetracycline): The Classic Chelators
The story of tetracyclines and zinc is a cautionary tale, one of the most well-documented examples of drug-nutrient interaction. These broad-spectrum antibiotics, commonly prescribed for conditions ranging from acne to respiratory tract infections and Lyme disease, are notorious for their ability to chelate with divalent and trivalent metal ions. Zinc, being a divalent cation (Zn²⁺), is a prime candidate for this molecular handshake.
When a tetracycline antibiotic is ingested alongside a zinc supplement, or even zinc-rich foods, the drug molecule forms a stable, insoluble complex with the zinc ion in the gastrointestinal tract. This complex is effectively "locked up," unable to pass through the intestinal wall into the bloodstream. The consequence is twofold: the absorption of the tetracycline antibiotic is significantly reduced, potentially compromising its ability to fight the infection, and simultaneously, the absorption of zinc is also diminished.
- The Recommendation: The standard advice, born from decades of clinical observation and research, is to space out the administration of tetracyclines and zinc supplements by at least 2 to 4 hours. This allows sufficient time for one substance to be absorbed before the other enters the digestive system, minimizing the chances of chelation. Ideally, take zinc after the antibiotic has been absorbed, or vice-versa, ensuring a substantial gap.
Fluoroquinolones (e.g., Ciprofloxacin, Levofloxacin, Moxifloxacin): A Similar Narrative
Another potent class of antibiotics, the fluoroquinolones, shares a similar, albeit sometimes less pronounced, propensity for chelation with metal ions. Used for a wide array of bacterial infections, including urinary tract infections, respiratory infections, and skin infections, these drugs can also bind to zinc, iron, calcium, and magnesium.
Like tetracyclines, when fluoroquinolones encounter zinc in the gastrointestinal tract, they can form insoluble chelates. This interaction leads to reduced absorption of both the antibiotic and the zinc. The clinical implications are identical: diminished therapeutic efficacy of the antibiotic and potential long-term zinc deficiency, particularly in individuals on prolonged courses or with pre-existing marginal zinc status.
- The Recommendation: Similar to tetracyclines, a spacing strategy is crucial. Fluoroquinolones should be taken at least 2 hours before or 6 hours after a zinc supplement. This broader window is often recommended due to variations in absorption rates and the critical importance of ensuring optimal antibiotic efficacy.
The "story" here is about the unintended consequences of potent medicines. While designed to combat invaders, their chemical properties can inadvertently create internal conflicts, reminding us that even beneficial interventions require careful consideration of their interactions within the body’s complex ecosystem.
Chapter 2: The Acid Suppressors – A Double-Edged Sword
Our stomachs are acidic powerhouses, designed to break down food and kill pathogens. This acidic environment, however, is also crucial for the solubility and subsequent absorption of many nutrients and drugs. When this environment is altered by acid-suppressing medications, a new kind of tangle can emerge for zinc.
Proton Pump Inhibitors (PPIs) (e.g., Omeprazole, Pantoprazole, Esomeprazole): The pH Paradox
Proton Pump Inhibitors are incredibly effective drugs, widely used to treat conditions like gastroesophageal reflux disease (GERD), peptic ulcers, and Zollinger-Ellison syndrome. They work by irreversibly blocking the proton pumps in the stomach lining, dramatically reducing gastric acid production. While excellent for alleviating acid-related symptoms, this profound reduction in stomach acid (leading to a higher, less acidic pH) can have downstream effects on nutrient absorption.
Zinc, like several other minerals (e.g., iron, calcium, magnesium), requires an acidic environment to be optimally dissolved and converted into a form that can be efficiently absorbed in the small intestine. When the stomach’s acidity is suppressed by PPIs, especially with long-term use, the solubility of zinc can be reduced. This means less zinc is available in a form that the intestinal transporters can recognize and absorb, potentially leading to chronic, subclinical zinc deficiency.
- The Recommendation: While direct chelation isn’t the primary mechanism here, the impact on pH is significant. For individuals on long-term PPI therapy, or those taking high-dose zinc supplements, it’s advisable to discuss zinc supplementation with their healthcare provider. While spacing might help marginally, the fundamental issue is the altered stomach environment. Taking zinc with food, which naturally stimulates some acid production, might offer a slight advantage. The more critical action is monitoring zinc levels and considering alternative forms of zinc (e.g., zinc citrate, picolinate, gluconate) which may be less dependent on gastric acid for absorption, though evidence on this specific aspect with PPIs is still evolving.
The "story" of PPIs and zinc highlights the delicate balance of the body’s internal chemistry. A solution to one problem (excess acid) can inadvertently create another (impaired nutrient absorption), underscoring the interconnectedness of physiological systems.
Chapter 3: Bone Builders and Mineral Movers – A Complex Dance
Some medications have a direct or indirect impact on mineral metabolism or absorption that extends beyond simple chelation, creating nuanced interactions with zinc.
Bisphosphonates (e.g., Alendronate, Risedronate, Ibandronate): Not Just for Bones
Bisphosphonates are cornerstones in the treatment and prevention of osteoporosis, working by inhibiting osteoclast activity (cells that break down bone). These drugs are known for their notoriously poor oral bioavailability, meaning only a small fraction of an orally administered dose is actually absorbed. This makes them highly susceptible to interference.
While the primary concern with bisphosphonates is usually their interaction with calcium, iron, and magnesium (which can form complexes and reduce bisphosphonate absorption), some research suggests that zinc, particularly in high doses, could also interfere with bisphosphonate absorption. The precise mechanism is not as clearly defined as with tetracyclines, but it likely involves some degree of competitive binding or complex formation that renders the bisphosphonate less available for absorption.
- The Recommendation: To ensure maximal absorption of the bisphosphonate, which is critical for its efficacy in bone health, these drugs are typically taken on an empty stomach with a full glass of plain water, at least 30 to 60 minutes before any food, beverages (other than plain water), or other medications/supplements. This strict protocol extends to zinc supplements as well. Adhering to this precise timing is paramount.
Therapeutic Chelating Agents (e.g., Penicillamine): The Intentional Zinc Drain
In stark contrast to inadvertent chelation, some drugs are designed to chelate metals. Penicillamine, for instance, is a potent chelating agent used to treat conditions like Wilson’s disease (a genetic disorder causing copper accumulation), and severe rheumatoid arthritis. Its therapeutic effect relies on its ability to bind to and remove excess heavy metals from the body.
The "tangle" here is not an accidental side effect but an inherent property of the drug. Penicillamine will readily bind to various metal ions, including copper, lead, mercury, and, crucially, zinc. While beneficial for removing toxic metals, its indiscriminate chelating action means it will also bind to and deplete essential zinc stores.
- The Recommendation: For patients on penicillamine, zinc supplementation might be necessary, but it must be carefully managed under strict medical supervision. The timing of zinc administration relative to penicillamine would need to be strategically spaced, often by several hours, to maximize the absorption of the zinc supplement while still allowing the penicillamine to exert its therapeutic effect on other target metals. Regular monitoring of zinc levels is essential to prevent severe deficiency.
The "story" here explores the nuanced world of targeted drug action and its broader impact. While some drugs inadvertently create problems, others intentionally manipulate mineral balance, demanding a deeply informed and careful approach to supplementation.
Chapter 4: The Fluid Regulators – Diuretics and the Zinc Drain
Our kidneys are sophisticated filters, meticulously regulating fluid and electrolyte balance. Diuretics, medications that increase urine output, are invaluable for managing conditions like hypertension, heart failure, and edema. However, by altering kidney function, some diuretics can inadvertently lead to increased excretion of essential minerals, including zinc.
Thiazide Diuretics (e.g., Hydrochlorothiazide, Chlorthalidone): The Hidden Loss
Thiazide diuretics are commonly prescribed for high blood pressure. They work by inhibiting sodium and chloride reabsorption in the distal convoluted tubule of the kidney, leading to increased excretion of water, sodium, and chloride. However, their action can also influence the excretion of other ions. Long-term use of thiazide diuretics has been associated with increased urinary excretion of zinc. The exact mechanism isn’t fully understood but is thought to involve altered tubular reabsorption of zinc or changes in zinc-binding proteins in the kidney. Over time, this chronic loss can contribute to zinc deficiency, particularly in vulnerable populations.
- The Recommendation: For individuals on long-term thiazide diuretic therapy, especially those with other risk factors for zinc deficiency (e.g., elderly, poor diet, chronic disease), monitoring zinc levels and considering a zinc supplement may be prudent. Spacing zinc supplements from the diuretic might not prevent the increased urinary excretion, but it ensures optimal absorption of the supplemented zinc. Consultation with a healthcare provider is essential to determine if supplementation is warranted and at what dose.
Loop Diuretics (e.g., Furosemide, Bumetanide): Less Pronounced, Still a Factor
Loop diuretics are more potent than thiazides and are typically used for more severe fluid retention. While they primarily affect the loop of Henle, their impact on zinc excretion is generally considered less significant or consistent than that of thiazide diuretics. However, some studies have shown an increase in urinary zinc loss with chronic loop diuretic use as well.
- The Recommendation: Similar to thiazides, long-term use warrants consideration of zinc status. While a direct "timing tangle" at the absorption level is not the primary concern, the potential for increased urinary loss means that ensuring adequate zinc intake, potentially through supplementation, might be necessary. As always, medical guidance is advised.
The "story" of diuretics and zinc highlights the kidney’s role as a sophisticated regulator and the potential for even well-intentioned interventions to subtly alter mineral balance over time. It underscores the importance of a holistic view of patient care, looking beyond the primary therapeutic effect to the broader physiological impact.
Chapter 5: The Thyroid’s Tune and Iron’s Might – Other Notable Interactions
The tapestry of drug-nutrient interactions is vast, and while some are highly specific, others arise from broader physiological principles or common co-supplementation practices.
Thyroid Hormones (e.g., Levothyroxine): The Delicate Balance
Levothyroxine is a synthetic thyroid hormone, crucial for individuals with hypothyroidism. Its absorption is highly sensitive to various factors, including food, other medications, and supplements. Some evidence suggests that certain minerals, including zinc, could potentially interfere with levothyroxine absorption if taken concurrently. The mechanism might involve chelation or altered pH, similar to other mineral interactions, though it’s less definitively established than for calcium or iron.
- The Recommendation: Levothyroxine is typically advised to be taken on an empty stomach, at least 30 to 60 minutes before breakfast and other medications/supplements, to ensure optimal and consistent absorption. This general rule should extend to zinc supplements to avoid any potential, even if minor, interference with thyroid hormone efficacy.
High-Dose Iron Supplements: The Competitive Edge
This interaction is less about a drug and more about a common nutrient supplement. Iron and zinc are both essential divalent cations, and they share some common absorption pathways in the small intestine. When high doses of iron supplements (e.g., for iron-deficiency anemia) are taken concurrently with zinc supplements, they can compete for these limited transporters.
This competition can lead to reduced absorption of both minerals, but it is often more pronounced for zinc, as iron’s binding affinity can sometimes be higher or its concentration in supplements much greater. This is a particularly common "timing tangle" for individuals who are conscientiously trying to address multiple nutritional deficiencies.
- The Recommendation: To minimize competition, iron and zinc supplements should ideally be taken at different times of the day, spaced by at least 2 to 3 hours. If both are absolutely necessary, prioritize the timing based on the most critical deficiency or the one requiring maximal absorption. For example, if severe iron deficiency anemia is present, ensure maximal iron absorption first, then space zinc. Taking these supplements with food can also slightly reduce competition by slowing down absorption, but spacing remains the most effective strategy.
The "story" here illustrates the interconnectedness of bodily systems and the common pitfalls of multi-supplement usage without proper guidance. Even beneficial nutrients can become antagonists if their absorption pathways are not respected.
Decoding the Signals: Practical Strategies and Risk Factors
Navigating the complex landscape of drug-nutrient interactions requires not just knowledge but also a practical, proactive approach. For the knowledgeable individual, understanding the "timing tangle" is the first step; implementing strategies to avoid it is the crucial second.
The Golden Rule: Spacing Out
As evident throughout our exploration, the most universally applicable and effective strategy to mitigate most drug-zinc interactions is strategic spacing. By allowing sufficient time for one substance to be absorbed before the other is introduced, you minimize the chances of chelation, competition, or other forms of interference. The specific time interval varies depending on the drug and its pharmacokinetics, but generally, a window of 2 to 4 hours (and sometimes even longer for specific drugs like fluoroquinolones or bisphosphonates) is recommended. Always err on the side of caution and consult specific drug information or your pharmacist.
The Indispensable Guide: Consultation with Healthcare Professionals
While this article provides detailed information, it is not a substitute for personalized medical advice. Your pharmacist and physician are your most valuable resources. They have access to your full medication history, understand your specific health conditions, and can provide tailored advice.
- Pharmacist: Pharmacists are experts in drug interactions. When picking up new prescriptions or considering a supplement, always inform your pharmacist about all other medications (prescription, over-the-counter) and supplements you are taking.
- Physician: Discuss your zinc supplementation plans with your doctor, especially if you are on long-term medications or have chronic health conditions. They can assess your overall nutritional status and determine if zinc supplementation is truly necessary and safe in your specific context.
Monitoring for Deficiency: Decoding the Body’s Signals
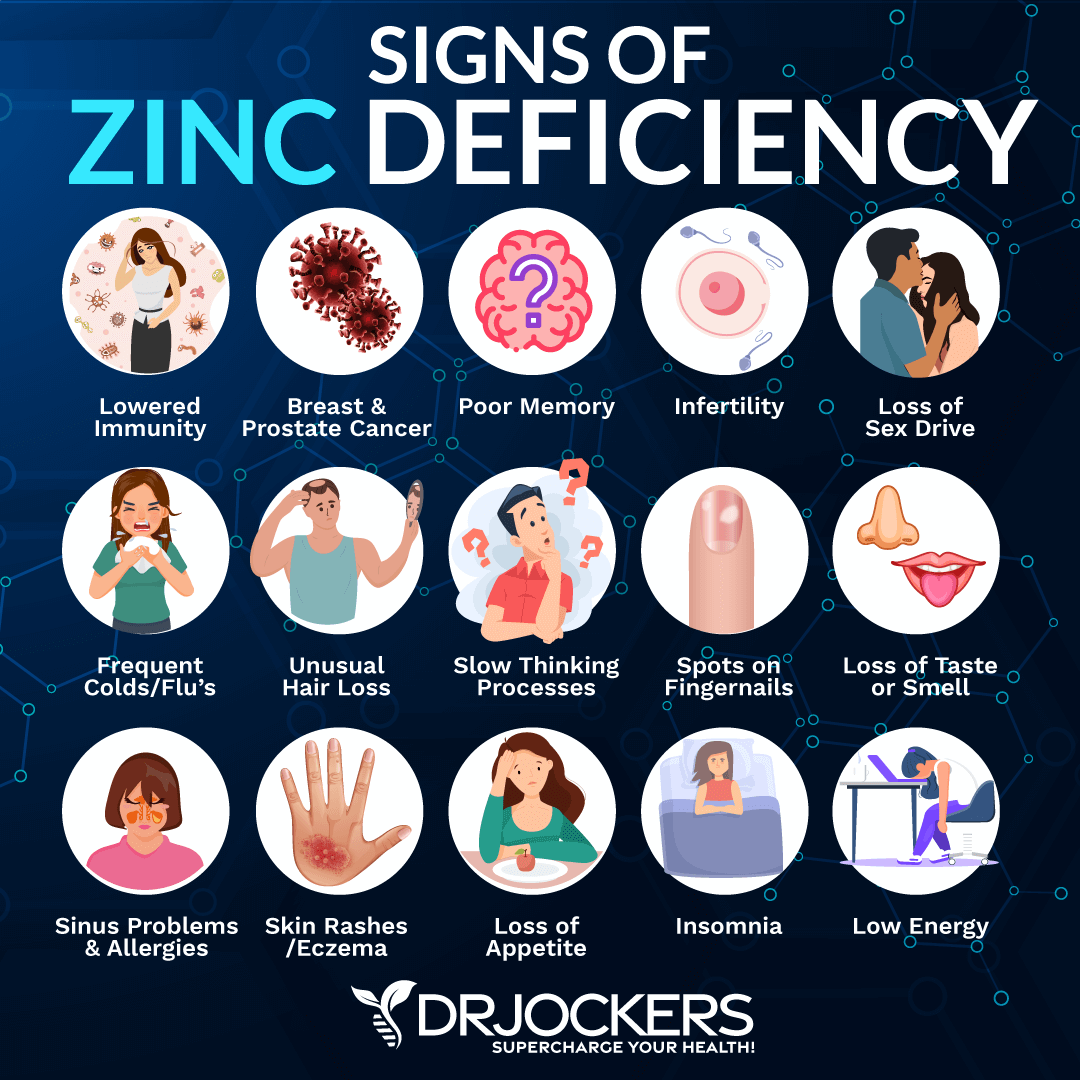
For individuals at higher risk of zinc deficiency due to long-term medication use, it’s important to be aware of the signs and symptoms. These can be subtle and non-specific but may include:
- Frequent infections
- Slow wound healing
- Hair loss
- Skin lesions
- Diarrhea
- Loss of appetite
- Impaired taste or smell
- Mood disturbances
If you experience these symptoms while on medications known to interact with zinc, discuss the possibility of testing your zinc levels with your doctor.
Who is Most Vulnerable? Identifying Risk Factors
Certain populations are inherently more susceptible to experiencing negative consequences from drug-zinc interactions:
- Elderly Individuals: Often on multiple medications (polypharmacy), may have reduced dietary intake of zinc, and can have age-related decline in absorption efficiency.
- Individuals with Chronic Diseases: Conditions like kidney disease, inflammatory bowel disease, or diabetes can independently affect zinc status.
- Those on Long-Term Medication Regimens: The cumulative effect of interactions over months or years can lead to significant deficiencies.
- Individuals with Poor Dietary Habits: A diet already low in zinc-rich foods (e.g., meat, shellfish, legumes, nuts) leaves less buffer against drug-induced depletion.
- Individuals Taking Multiple Supplements: The risk of competitive absorption (e.g., high-dose iron with zinc) increases with the number of supplements taken concurrently.
Being aware of these risk factors empowers you to be more vigilant in managing your medication and supplement timing.
Mastering the Maze: Empowering Informed Choices
The journey through the "timing tangle" of zinc and common medications reveals a landscape far more intricate than often perceived. It underscores a fundamental truth: the human body is a marvel of interconnected systems, where even seemingly isolated interventions can send ripples through the entire physiological orchestra. The dance between drugs and nutrients is not always harmonious; sometimes, it’s a clash of titans or a subtle undermining of critical processes.
For the knowledgeable individual, this understanding is not a source of anxiety but a wellspring of empowerment. It transforms the act of taking a pill or a supplement from a routine gesture into a mindful, informed decision. By recognizing the mechanisms of chelation, competition, pH alteration, and altered excretion, you gain the ability to anticipate potential conflicts and implement effective strategies to circumvent them.
The ultimate takeaway is clear: Never underestimate the importance of timing and communication. Space out your supplements and medications diligently, adhering to the recommended intervals. More importantly, engage in open and honest dialogue with your healthcare providers – your doctor and pharmacist. They are your navigators in this complex maze, possessing the expertise to guide you toward optimal health outcomes, ensuring that your medications work as intended and your nutritional well-being remains robust.
In mastering this timing tangle, you are not just preventing adverse interactions; you are actively participating in the sophisticated choreography of your own health, ensuring that every element, from the most potent drug to the tiniest trace mineral, performs its role in perfect harmony. This is the essence of informed self-care: a commitment to understanding, vigilance, and proactive management in the pursuit of sustained wellness.
:max_bytes(150000):strip_icc()/health-best-zinc-supplements-tout-d0cf8e2ff81a4e1ab2a8c7d7ab095e9b.jpg)