The Unseen Architect: Why Zinc, Our Unsung Hero, Demands Respect in the Pharmaceutical Symphony (The Essential Spacing Guide)
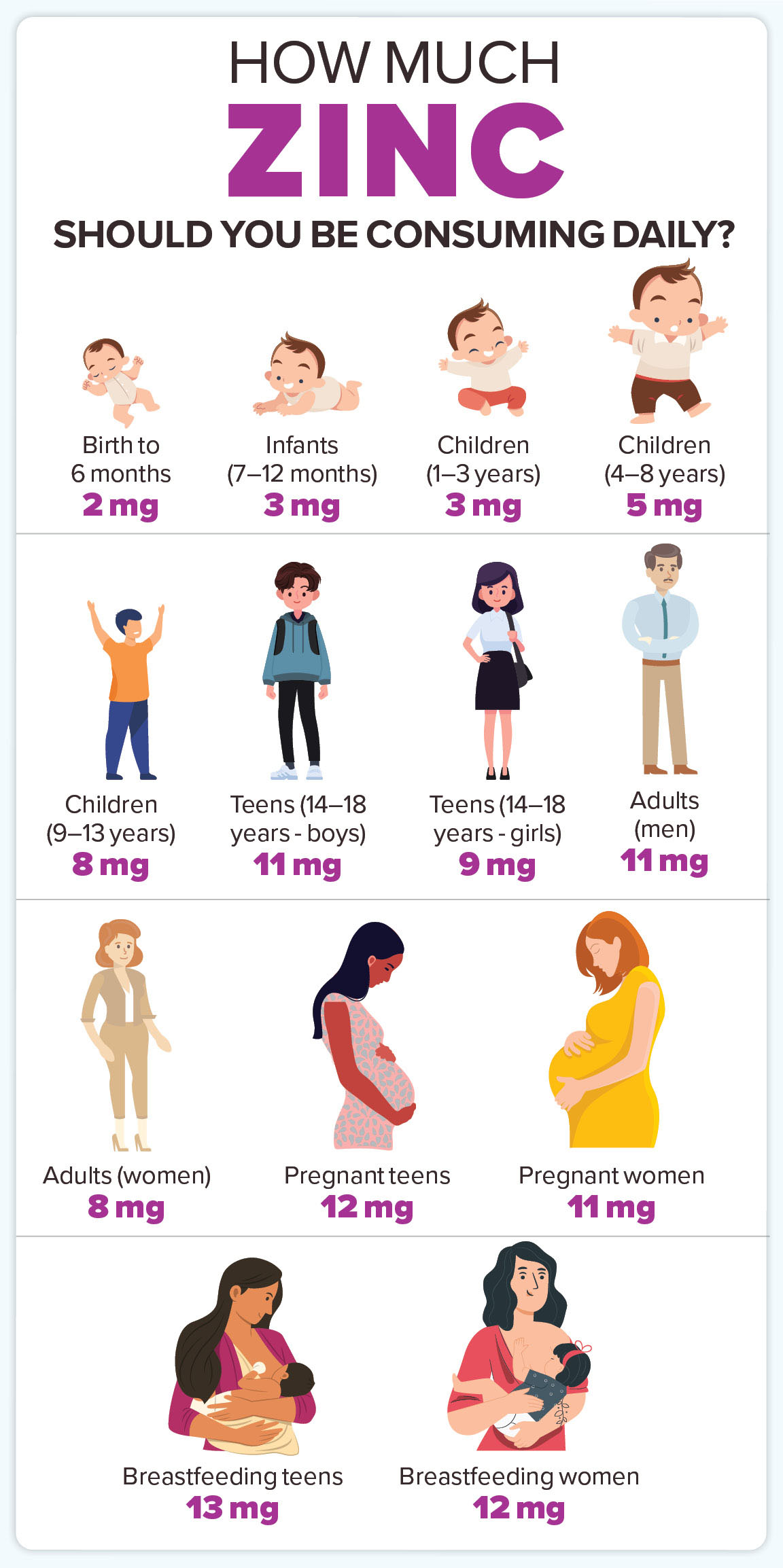
The hum of the pharmacy, a symphony of hushed conversations and the soft rustle of prescription bags, often masks a far more intricate ballet playing out at a molecular level within our bodies. We trust the white-coated professionals, the rigorous science behind each pill, each supplement. We strive to be diligent, to optimize our health, to fight off every sniffle and ward off every illness. And in this pursuit, zinc, the unassuming mineral, often steps onto the stage as an unsung hero. Essential for immune function, wound healing, DNA synthesis, and even our sense of taste and smell, zinc is a cornerstone of good health.
But what if this very dedication, this proactive approach to wellness, inadvertently created a discordant note in our body’s delicate biochemical orchestra? What if the seemingly innocuous act of taking a zinc supplement alongside a crucial medication could undermine both, turning a gesture of self-care into a silent sabotage?
This is not a story of malicious intent or egregious error, but one of intricate chemistry, of molecular magnetism, and the profound importance of timing. It’s a narrative for the knowledgeable, for those who seek to understand the deeper currents flowing beneath the surface of health advice, for the meticulous few who wish to orchestrate their wellness with precision. This is the story of why zinc, our indispensable ally, demands respect, and a precise spacing guide, when sharing the stage with certain powerful pharmaceutical players.
The Essential Mineral: A Double-Edged Sword of Wellness
Before we delve into the potential pitfalls, let’s appreciate the star of our show: zinc. This trace element is a veritable molecular maestro, orchestrating over 300 enzymatic reactions in the human body. Its influence permeates nearly every aspect of our physiological well-being:
- Immune Guardian: Zinc is critical for the development and function of immune cells, playing a pivotal role in both innate and adaptive immunity. From bolstering T-cell activity to enhancing natural killer cell function, it’s our body’s internal security guard. This is why it’s a popular go-to for warding off colds and flu.
- Wound Healer: It’s indispensable for cell growth and division, making it vital for tissue repair and wound healing. Scars fade faster, cuts close more efficiently when zinc is abundant.
- Sensory Enhancer: Zinc is a component of gustin, a protein essential for taste bud development. A deficiency can literally dull the flavors of life and impair our sense of smell.
- Antioxidant Defender: It helps reduce oxidative stress by acting as a cofactor for superoxide dismutase (SOD), a powerful antioxidant enzyme.
- DNA and Protein Synthesizer: Essential for the intricate processes of DNA replication and protein synthesis, it’s fundamental to growth, development, and cellular maintenance.
Given this impressive resume, it’s no wonder that millions reach for zinc supplements, especially during cold and flu season, or when feeling run down. It’s an accessible, seemingly benign way to bolster health. But here lies the paradox: the very properties that make zinc so essential – its ability to bind to proteins, its ionic charge – are precisely what can turn it into a silent saboteur when introduced alongside specific medications.
The core mechanism of interaction often revolves around chelation. Imagine zinc as a tiny, highly attractive magnet. Certain drug molecules are also like magnets, and when they meet zinc in the acidic environment of the stomach or the alkaline milieu of the small intestine, they can form an insoluble, inert complex. This complex is too large or too stable to be absorbed effectively into the bloodstream. The result? Neither the zinc nor the drug reaches its intended target in sufficient quantities, rendering both less effective.
It’s a chemical tango where the partners become so intertwined they can no longer perform their individual roles.
The Silent Saboteurs: Key Drug Classes and Their Dance with Zinc
Our story now shifts to the pharmaceutical characters who, through their own biochemical needs and structures, enter into this precarious dance with zinc. These are not interactions of toxicity, but of efficacy – where the effectiveness of life-saving or crucial medications is diminished, potentially leading to treatment failure.
1. The Antibiotics: When Molecular Magnets Collide
This is perhaps the most critical and well-documented interaction. Two major classes of antibiotics are particularly vulnerable to zinc’s chelating embrace: Tetracyclines and Quinolones (Fluoroquinolones).
a) Tetracyclines (e.g., Doxycycline, Minocycline, Tetracycline itself)
- The Scenario: Sarah, a diligent college student, develops a persistent cough. Her doctor prescribes doxycycline for a suspected bacterial infection. Anxious to recover quickly, and having read about zinc’s immune-boosting properties, she also takes her daily zinc supplement alongside her antibiotic.
- The Interaction: Tetracyclines are broad-spectrum antibiotics used to treat a wide array of bacterial infections, from respiratory tract infections and UTIs to acne and Lyme disease. Their molecular structure contains functional groups that are highly adept at binding to multivalent cations like zinc (Zn2+), calcium (Ca2+), iron (Fe2+/Fe3+), and magnesium (Mg2+). When doxycycline and zinc meet in the gastrointestinal tract, they form an insoluble chelate.
- The Consequence: This chelate cannot be efficiently absorbed through the intestinal wall into the bloodstream. The result is a significant reduction in the bioavailability of both the antibiotic and the zinc. Sarah’s infection might not clear, leading to prolonged illness, potential complications, and the need for a different course of treatment. The doxycycline, designed to be a potent weapon, becomes largely inert.
- The Essential Spacing Guide: The general recommendation is to separate tetracycline antibiotics and zinc supplements by at least 2 to 4 hours. Some sources even suggest a wider gap, up to 6 hours, to be absolutely safe, especially if the patient has compromised gut motility. Taking the antibiotic first, allowing it to be absorbed, and then the zinc, or vice versa, helps ensure that their paths don’t cross during the critical absorption window.
b) Quinolones / Fluoroquinolones (e.g., Ciprofloxacin, Levofloxacin, Moxifloxacin)
- The Scenario: Mark, an active retiree, develops a urinary tract infection. His doctor prescribes ciprofloxacin, a powerful antibiotic. Mark, ever health-conscious, has been taking zinc for years to support his prostate health. He takes both with his morning routine.
- The Interaction: Fluoroquinolones are another class of broad-spectrum antibiotics, highly effective against a range of bacterial infections, including UTIs, respiratory infections, and skin infections. Like tetracyclines, their chemical structure contains specific groups that readily chelate with multivalent cations like zinc. The binding affinity is strong, forming stable, unabsorbable complexes.
- The Consequence: The interaction leads to a significant decrease in the absorption and efficacy of the fluoroquinolone. Mark’s UTI may persist or worsen, potentially leading to a more severe infection, antibiotic resistance, and the need for more aggressive or prolonged treatment. The antibiotic’s ability to reach therapeutic concentrations in the bloodstream is severely hampered.
- The Essential Spacing Guide: Similar to tetracyclines, fluoroquinolones and zinc supplements should be separated by at least 2 to 4 hours. Again, taking the antibiotic first, allowing for absorption, then the zinc, or vice versa, is the key. Given the critical nature of these antibiotics in treating serious infections, strict adherence to this spacing is paramount.
Dr. Anya Sharma, a seasoned infectious disease specialist, recounts a hypothetical but all-too-common scenario: "We see patients returning with persistent infections, sometimes even worsening, despite being on the ‘right’ antibiotic. Often, a deeper dive into their medication regimen reveals they’ve been inadvertently undermining their treatment by taking a supplement like zinc too close to their antibiotics. It’s a frustrating situation because the patient’s intentions are good, but the biochemical reality is unforgiving."
2. The Diuretics: Accelerating Zinc’s Exit
Not all interactions involve direct chelation in the gut. Some drugs influence zinc levels by altering its excretion from the body.
a) Thiazide Diuretics (e.g., Hydrochlorothiazide)
b) Loop Diuretics (e.g., Furosemide, Bumetanide)
- The Scenario: Eleanor, managing high blood pressure and mild edema, takes hydrochlorothiazide daily. Over several months, she starts to feel unusually fatigued, her sense of taste seems off, and she notices her hair thinning. She wonders if it’s just age.
- The Interaction: Diuretics, commonly prescribed for high blood pressure, heart failure, and edema, work by increasing the excretion of sodium and water from the body. However, some, particularly thiazide and loop diuretics, can also increase the urinary excretion of other essential minerals, including zinc. While not an acute interaction like chelation, long-term use can lead to a gradual depletion of the body’s zinc stores.
- The Consequence: Chronic zinc depletion can manifest in a variety of symptoms, including impaired immune function, delayed wound healing, hair loss, skin lesions, and altered taste perception – precisely the issues Eleanor is experiencing. This isn’t about reduced drug efficacy, but rather about the drug’s long-term impact on the body’s nutrient status.
- The Essential Spacing Guide: For diuretics, the interaction is less about immediate competition for absorption and more about long-term metabolic balance. There isn’t a strict "spacing" rule in the same way as antibiotics. Instead, the focus shifts to monitoring and potential supplementation. If on long-term diuretic therapy, especially loop or thiazide diuretics, patients should discuss their zinc status with their doctor. Regular monitoring of zinc levels might be advisable, and a doctor may recommend a zinc supplement, taken at a different time of day than the diuretic, or as part of a comprehensive multivitamin. The goal here is to prevent deficiency, not to avoid a direct binding interaction.
3. Chelating Agents: The Zinc Magnets with a Purpose
Some drugs are designed to chelate metals, and zinc is often among their targets.
a) Penicillamine (e.g., Cuprimine, Depen)
- The Scenario: A patient with Wilson’s disease, a genetic disorder causing copper accumulation, is prescribed penicillamine to help remove excess copper from their body.
- The Interaction: Penicillamine is a potent chelating agent used therapeutically to bind and facilitate the excretion of heavy metals, particularly copper in Wilson’s disease, but also lead, mercury, and, importantly, zinc. It’s designed to form stable complexes with these metal ions, rendering them excretable.
- The Consequence: While effective for its intended purpose, penicillamine’s non-specific chelating action means it can also deplete the body of essential metals like zinc. This can lead to severe zinc deficiency if not managed carefully, potentially causing immune suppression, skin problems, and other adverse effects.
- The Essential Spacing Guide: In this context, zinc supplementation might actually be necessary to counteract the drug’s depleting effect, but it must be meticulously managed. Penicillamine and zinc supplements should be taken at least 2 hours apart, and ideally at different major meals or times of the day, to minimize direct competition and allow each compound to perform its function without mutual interference. The decision to supplement and the dosage should be under strict medical supervision.
4. Other Noteworthy Considerations: The Long Game
While not as acutely problematic as the antibiotic interactions, other medications can have long-term implications for zinc status:
- Proton Pump Inhibitors (PPIs) (e.g., Omeprazole, Pantoprazole): These drugs reduce stomach acid. While beneficial for GERD and ulcers, prolonged reduction in stomach acid can impair the absorption of various nutrients, including zinc, which requires an acidic environment for optimal absorption. This is a long-term concern rather than an immediate interaction, potentially leading to chronic zinc depletion. Monitoring and perhaps a spaced supplement might be considered over time.
- Certain Chemotherapy Agents (e.g., Cisplatin): Some chemotherapy drugs can lead to increased zinc excretion or altered zinc metabolism, contributing to zinc deficiency in cancer patients. Again, this is a long-term management issue, requiring medical oversight for potential supplementation.
The Essential Spacing Guide: A Practical Compass
The crux of managing these interactions lies in intelligent timing. It’s about respecting the molecular choreography, ensuring each player gets its moment on stage without jostling for position.
General Principles of Spacing:
- Understand the Mechanism: Is it chelation in the gut (most common for antibiotics) or altered excretion/absorption over time? This dictates the strategy.
- Prioritize the Critical Drug: If one drug is life-saving (like an antibiotic for a serious infection), its absorption takes precedence.
- The "2-4 Hour Rule": For drugs that directly chelate zinc (Tetracyclines, Quinolones, Penicillamine), a separation of at least 2 to 4 hours is the gold standard.
- How to implement: If you take your antibiotic at 8:00 AM, take your zinc no earlier than 10:00 AM, and preferably later (e.g., 12:00 PM or 2:00 PM), or with a completely different meal.
- Why it works: This time window allows the first substance to largely pass through the stomach and be absorbed into the small intestine before the second substance arrives. By then, the critical binding sites might be unavailable, or the drug might already be in the bloodstream, preventing the formation of an unabsorbable complex.
- Consistency is Key: Once a spacing routine is established, stick to it. Erratic timing can negate the benefits.
- Listen to Your Body, But Consult a Professional: While symptoms of deficiency or reduced drug efficacy can be subtle, any changes should prompt a conversation with your healthcare provider.
Specific Spacing Recommendations (Recap):
- Tetracycline Antibiotics (Doxycycline, Minocycline): Separate by 2 to 4 hours.
- Quinolone Antibiotics (Ciprofloxacin, Levofloxacin): Separate by 2 to 4 hours.
- Penicillamine: Separate by 2 hours (and monitor zinc levels closely under medical guidance).
- Diuretics (Hydrochlorothiazide, Furosemide): Less about spacing, more about long-term monitoring and potential supplementation as advised by a doctor. If supplementing, space from the diuretic as a general good practice.
- Proton Pump Inhibitors (Omeprazole, Pantoprazole): For long-term use, discuss with your doctor about potential zinc deficiency. If supplementing, take zinc at a different time of day, ideally with food, to aid absorption.
Imagine a patient, Emily, who needs levofloxacin for a sinus infection and takes a daily zinc supplement. Her prescription reads: "Take levofloxacin once daily." If she takes her antibiotic at 8:00 AM, her zinc should be taken no earlier than 10:00 AM, or even better, with her dinner at 6:00 PM. This simple shift in timing ensures that both the antibiotic and the zinc can perform their respective duties unimpeded. It’s a small adjustment with potentially profound implications for treatment success and overall well-being.
Beyond Spacing: A Holistic Approach to Nutrient-Drug Harmony
The story of zinc and drug interactions is not just about timing; it’s about a broader philosophy of informed patient care and communication.
- Full Disclosure is Non-Negotiable: Every healthcare professional – your doctor, your pharmacist, your dietitian – needs a complete picture of everything you put into your body. This includes prescription medications, over-the-counter drugs, herbal remedies, and all dietary supplements. Do not assume something is "just a vitamin" and therefore inconsequential. The pharmacist, often the unsung guardian at the gates of medication safety, is an invaluable resource for checking potential interactions.
- Dietary Zinc vs. Supplemental Zinc: While dietary sources of zinc (red meat, poultry, beans, nuts, certain seafood) can also chelate with some drugs, the concentration of zinc in a typical supplement (often 15-50 mg) is significantly higher than what you’d get from a single meal. Therefore, the risk of clinically significant interaction is far greater with supplements. However, maintaining a balanced diet remains crucial for overall health.
- Personalized Medicine: Every individual is unique. Factors like gut motility, gastric pH, kidney function, and liver metabolism can influence how drugs and nutrients are absorbed and processed. What works for one person might need slight adjustment for another. This underscores the need for personalized advice from healthcare providers.
- Monitoring and Awareness: Be vigilant for signs that your medication isn’t working as expected, or for symptoms of nutrient deficiency (fatigue, hair loss, impaired immunity, altered taste). These could be subtle indicators that an interaction is at play.
- The Evolving Science: The field of drug-nutrient interactions is dynamic. New research continually emerges, refining our understanding. What we know today might be nuanced tomorrow. Staying informed, through reliable sources and ongoing dialogue with your medical team, is key.
Conclusion: The Power of Knowledge, The Precision of Care
The tale of zinc and its interactions with various drugs is a compelling reminder that the human body is a complex, interconnected system, and every input, however seemingly small, can ripple through its intricate networks. Zinc, a vital mineral, capable of bolstering our immune defenses and promoting healing, also possesses a molecular personality that demands respect and careful consideration when paired with certain pharmaceutical agents.
The essential spacing guide is more than a set of rules; it’s a testament to the power of knowledge, the precision of care, and the proactive spirit of a knowledgeable audience. By understanding the "why" behind these interactions – the silent chelation, the altered excretion – we empower ourselves to navigate the modern landscape of health with greater wisdom and intention.
It’s about transforming a potential biochemical clash into a harmonious partnership, ensuring that every element, from the life-saving antibiotic to the essential trace mineral, gets its rightful moment to shine, contributing to the symphony of optimal health. So, the next time you reach for that zinc supplement, pause. Consider the other players on your daily health stage. A moment of informed reflection, a simple adjustment in timing, can be the difference between a silent sabotage and a powerful, orchestrated success.