The Science of the Ache: What Actually Happens in Your Brain During a Headache
The ache. It’s a primal, universal experience, a shadow that falls across the day, disrupting focus, stealing joy, and sometimes, bringing life to a grinding halt. For many, a headache is a mundane inconvenience, a mere blip on the radar of existence. Take an ibuprofen, drink some water, and carry on. But for those who know the true tyranny of the throbbing, the piercing, the relentless pressure, it is a profound and intimate betrayal. It is the brain, the very seat of consciousness, turning against itself, a tempest brewing within the skull.
To the uninitiated, a headache is just… pain. But for the discerning mind, the knowledgeable audience seeking to peer behind the veil of subjective suffering, we embark on a journey. A journey into the intricate, bewildering landscape of the human brain, to uncover the hidden symphony of cellular chaos, the misfiring neurons, and the delicate neurochemical dance that culminates in that all-too-familiar, unwelcome sensation. This is not just a biological exposition; it is the story of a brain under siege, a narrative woven from the threads of neuroscience, physiology, and the very fabric of human experience.
The Prelude: Whispers Before the Storm (Prodrome and Aura)
The headache, particularly the enigmatic migraine, rarely strikes without warning. Long before the hammer blow of pain descends, the brain often broadcasts subtle signals, a series of whispers that hint at the impending storm. This initial phase, known as the prodrome, is a testament to the brain’s complex and widespread involvement. It’s not just about pain; it’s about a systemic disruption.
Imagine the hypothalamus, that ancient, almond-sized conductor of the brain’s autonomic orchestra, stirring uneasily. Located deep within the brain, it governs our sleep-wake cycles, hunger, thirst, mood, and hormone release. During the prodrome, it may begin to falter, its delicate balance wavering. This can manifest as an inexplicable fatigue, a sudden craving for specific foods (often sweet or salty), irritability, depression, or an unusual sensitivity to light or sound. These aren’t just random symptoms; they are early signs of a brain preparing for battle, its homeostatic mechanisms already under strain. The hypothalamus, often acting as a central hub for initiating migraine attacks, begins to send out distress signals, setting the stage for the larger drama.
Following the prodrome, a more dramatic and visually arresting phenomenon can occur for about a third of migraine sufferers: the aura. This is where the story truly begins to unfold in a visually spectacular, albeit unsettling, manner. The most common aura is visual, manifesting as shimmering zigzags, flashing lights, blind spots, or distorted perceptions. But auras can also be sensory (tingling, numbness), motor (weakness), or even affect speech.
At the heart of the aura lies a phenomenon known as Cortical Spreading Depression (CSD). Picture a ripple in a pond, but instead of water, it’s a wave of intense neuronal activity, followed by a prolonged period of silence, sweeping across the cerebral cortex. This wave of depolarization, where neurons rapidly fire in unison, then fall into a state of inhibited activity, is a profound energetic event. It begins, typically, in the visual cortex at the back of the brain and propagates forward at a slow, deliberate pace – about 2-5 millimeters per minute. As this wave washes over different cortical regions, it temporarily disrupts their normal function, leading to the transient neurological symptoms of the aura.
The CSD is like the initial tremor before a volcanic eruption. It involves a massive efflux of potassium ions from neurons, an influx of calcium, and the release of excitatory neurotransmitters like glutamate. This biochemical cascade is energy-intensive and can trigger inflammatory changes in the surrounding tissue. Crucially, as the CSD wave propagates, it activates the meningeal nociceptors – the pain-sensing nerve endings that drape over the brain’s surface. It’s this activation, a silent alarm bell ringing in the brain’s protective layers, that truly signals the onset of the main event: the pain.
The Genesis of the Ache: The Trigeminal Tango
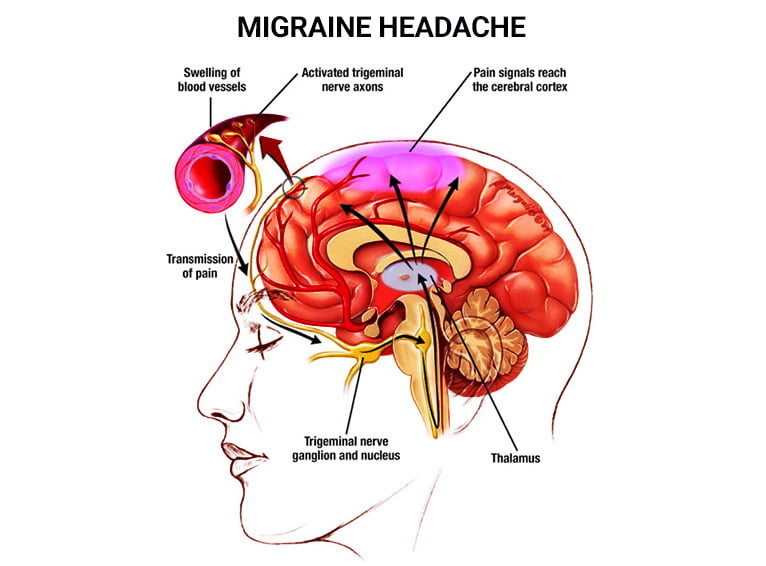
With the stage set by the prodrome and the initial spark of CSD, the narrative shifts to the direct genesis of the pain itself. This is where the trigeminal nervous system takes center stage, an intricate network responsible for sensation in the face, head, and brain’s protective coverings.
Imagine the meninges – the three layers of membrane (dura, arachnoid, pia mater) that encase the brain and spinal cord, acting as a crucial protective barrier. These layers are richly supplied with nociceptors, specialized nerve endings that detect potentially damaging stimuli. Unlike the brain tissue itself, which is devoid of pain receptors, these meningeal coverings are exquisitely sensitive. The blood vessels embedded within the dura mater, in particular, are densely innervated by fibers from the trigeminal nerve.
When the CSD wave passes over the meninges, or when other triggers (stress, hormonal shifts, environmental factors) initiate a cascade, these trigeminal nerve endings are activated. This activation is not a simple "on/off" switch; it’s a complex neuroinflammatory process. The activated trigeminal fibers release a potent cocktail of neuropeptides into the surrounding tissue, primarily Calcitonin Gene-Related Peptide (CGRP), but also Substance P and neurokinin A.
CGRP is a key protagonist in our story. It’s a powerful vasodilator, meaning it causes blood vessels to widen. While the role of vasodilation in migraine pain is a subject of ongoing debate (it’s no longer considered the sole cause of pain, but a contributing factor), its release contributes to a sterile inflammation. This neurogenic inflammation involves mast cells, which release histamine and other inflammatory mediators, further sensitizing the nerve endings. The meningeal blood vessels become leaky, allowing fluid and inflammatory cells to extravasate, creating a localized inflammatory soup that irritates and excites the already sensitized trigeminal nerve fibers.
The pain signal, now robust and insistent, travels along these trigeminal nerve fibers. These fibers converge at the trigeminal ganglion, a cluster of nerve cell bodies located near the brainstem. From here, the first-order neurons relay the signal to the trigeminal nucleus caudalis (TNC), located in the brainstem. The TNC is the primary relay station for pain signals from the face and head, a critical gatekeeper that determines which signals are amplified and which are suppressed.
At the TNC, a crucial process of peripheral sensitization begins. Repeated or intense stimulation of the trigeminal nerve endings in the meninges makes them more responsive to subsequent stimuli. Even normally innocuous pulsations of blood vessels or changes in intracranial pressure, which wouldn’t register as pain under normal circumstances, now become excruciating. This explains the characteristic throbbing quality of many headaches – each heartbeat sending a fresh wave of irritation through the hypersensitive meningeal nerves.
From Signal to Sensation: The Ascending Pathways
Once the pain signal has reached the TNC, its journey is far from over. It must now ascend through the brainstem and ultimately reach the higher cortical centers where it is consciously perceived and interpreted as pain. This is where the raw sensory data transforms into the subjective experience of suffering.
From the TNC, second-order neurons cross the midline and ascend to the thalamus, often referred to as the brain’s "grand central station" or relay hub. The thalamus acts as a sophisticated filter and distributor of sensory information, including pain. It decides which signals are important enough to be passed on to the cerebral cortex for conscious awareness. In the context of a headache, the thalamus is overwhelmed, struggling to filter the barrage of incoming pain signals.
From the thalamus, the signals are then projected to various regions of the cerebral cortex, forming a distributed network often referred to as the "pain matrix." This isn’t a single, isolated area, but a symphony of interconnected regions, each contributing to different facets of the pain experience:
- Somatosensory Cortex (S1 and S2): Located in the parietal lobe, these areas are responsible for localizing the pain and processing its intensity and quality (e.g., throbbing, sharp, dull). This is where the brain constructs a map of the body and identifies where the pain is coming from.
- Insula: Deep within the lateral sulcus, the insula plays a crucial role in integrating sensory, emotional, and visceral information. It contributes to the subjective feeling of "unpleasantness" and the gut-wrenching nausea often associated with severe headaches. It helps the brain understand the impact of the pain on the body’s internal state.
- Anterior Cingulate Cortex (ACC): Part of the limbic system, the ACC is heavily involved in the emotional and motivational aspects of pain. It contributes to the suffering, distress, and the urge to escape the pain. This is where the pain becomes truly aversive, triggering feelings of anguish and despair.
- Prefrontal Cortex (PFC): This frontal lobe region is responsible for higher-order cognitive functions like planning, decision-making, and attention. During a headache, the PFC is often hijacked, making it difficult to concentrate, think clearly, or engage in complex tasks. The pain dominates the mental landscape, disrupting executive function.
- Amygdala: Also part of the limbic system, the amygdala processes fear and anxiety. Its activation contributes to the emotional distress and heightened state of alarm that often accompanies severe headaches.
This widespread cortical activation explains why a headache is so much more than just a physical sensation. It’s a holistic experience that invades cognition, emotion, and behavior. The brain isn’t just registering a signal; it’s interpreting, reacting, and suffering.
The Amplifiers and Modulators: A Battle for Control
As the pain signal ascends and spreads, the brain isn’t a passive recipient. It’s engaged in a furious battle for control, attempting to modulate the incoming pain signals. However, in the context of a severe headache, these modulatory systems can sometimes be overwhelmed or even contribute to the problem.
One of the most insidious developments during a prolonged headache is central sensitization. While peripheral sensitization occurs at the trigeminal nerve endings, central sensitization takes root in the TNC and other higher pain processing centers. Here, neurons become hyperexcitable, firing more readily and intensely to even minor stimuli. This is like turning up the volume knob on the pain signal to an unbearable level.
Central sensitization is the neurobiological basis for allodynia and hyperalgesia. Allodynia is when a normally non-painful stimulus (like light touch, brushing hair, or even the gentle pressure of glasses on the nose) becomes intensely painful. Hyperalgesia is an exaggerated response to a painful stimulus. This phenomenon explains why simply lying on a pillow or wearing a hat can become unbearable during a migraine. The brain’s pain pathways have been rewired, making them exquisitely sensitive to any input.
Simultaneously, the brain’s own sophisticated descending pain modulatory system attempts to fight back. Originating in brainstem regions like the periaqueductal gray (PAG), the locus coeruleus (LC), and the raphe nuclei, these pathways send signals down to the TNC, attempting to inhibit the transmission of pain signals. They release neurotransmitters like serotonin, norepinephrine, and endogenous opioids (endorphins) to dampen the incoming ache.
However, in the context of a severe headache, this system can be compromised. For example, during a migraine, there are often fluctuations in serotonin levels. A sudden drop in serotonin, following an initial release, might disinhibit pain pathways, making the brain more vulnerable to pain. The LC, which produces norepinephrine, and the raphe nuclei, which produce serotonin, are critical for maintaining the brain’s pain balance. When these systems are dysregulated, the brain loses its ability to effectively gate the pain signals, allowing them to flood the higher centers.
Furthermore, the initial neurogenic inflammation from CGRP release can be perpetuated by other cellular actors. Glial cells – the brain’s support cells, including astrocytes and microglia – are not mere bystanders. When activated by inflammation or neuronal distress, they can release pro-inflammatory cytokines, chemokines, and reactive oxygen species, further sensitizing neurons and contributing to a cycle of inflammation and pain. It’s a vicious feedback loop, where inflammation triggers pain, and pain triggers more inflammation, solidifying the headache’s grip.
Beyond Pain: The Concomitant Symphony of Suffering
A headache, particularly a migraine, is rarely a solitary sensation. It brings with it a retinue of miseries, a constellation of symptoms that transform it into a systemic illness. These accompanying symptoms – nausea, vomiting, photophobia (light sensitivity), phonophobia (sound sensitivity), and osmophobia (smell sensitivity) – are not incidental; they are deeply woven into the neurobiology of the headache experience.
Many of these symptoms stem from the deep brainstem structures that are intimately involved in pain processing. The TNC, where the trigeminal nerve first synapses, is anatomically close to nuclei involved in autonomic functions and sensory processing:
- Nausea and Vomiting: The trigeminal pain pathways interact with the nucleus tractus solitarius (NTS) and the area postrema in the brainstem. The NTS receives visceral sensory information (including from the gut), while the area postrema is a chemoreceptor trigger zone, sensitive to circulating toxins and involved in initiating vomiting. The intense pain signals from the TNC can activate these adjacent structures, leading to the profound gastrointestinal distress that so often accompanies severe headaches. The hypothalamus, again, plays a role in coordinating these autonomic responses.
- Photophobia and Phonophobia: The pathways from the TNC project not only to the thalamus and cortex but also to areas involved in visual and auditory processing, including the superior colliculus and the thalamic nuclei that relay visual and auditory information. During a headache, these pathways become hypersensitive. Normal light or sound, which would otherwise be ignored, is perceived as intensely irritating and painful. The brain’s filtering mechanisms for sensory input are compromised, turning everyday stimuli into unbearable assaults. Even the emotional centers, like the amygdala, can contribute to the aversion, associating light and sound with increased suffering.
- Osmophobia: Similarly, increased sensitivity to smells is thought to involve pathways connecting the trigeminal system with olfactory processing centers, possibly through interactions in the brainstem or higher cortical areas, making even pleasant aromas repulsive.
These concomitant symptoms underscore the pervasive nature of the headache within the brain. It’s not just a pain signal; it’s a disruption of the brain’s entire sensory and autonomic landscape, forcing the individual into a state of sensory deprivation and physiological distress.
The Aftermath: The Brain’s Slow Retreat (Postdrome)
Even after the intense throbbing subsides, the brain does not immediately return to normalcy. The "headache hangover," or postdrome, is a testament to the profound energetic and neurochemical upheaval the brain has endured. This phase can last for hours or even days, leaving the individual feeling drained, foggy, and often with residual tenderness in the scalp.
During the postdrome, the brain is essentially recovering from a massive physiological shock. The immense neuronal activity, the widespread inflammation, and the frantic attempts at pain modulation have depleted neurotransmitter stores, particularly serotonin and norepinephrine. The brain’s energy reserves have been significantly taxed.
Symptoms during this phase often include:
- Fatigue and Exhaustion: The brain is literally tired, having expended enormous energy during the attack.
- Cognitive Impairment: Difficulty concentrating, "brain fog," and mild memory issues are common as the prefrontal cortex and other cognitive centers slowly reset.
- Mood Disturbances: Residual irritability, sadness, or a general sense of malaise may linger as neurotransmitter levels rebalance.
- Muscle Soreness: Scalp and neck muscles, often tensed during the headache in an unconscious attempt to brace against the pain, can remain sore.
The postdrome is a crucial reminder that a headache is a whole-brain event, with repercussions that extend far beyond the immediate pain. It highlights the brain’s vulnerability and the time it requires to restore its delicate homeostasis after such a profound disturbance.
The Enigma of Chronic Headaches and Genetic Echoes
For some, the story of the headache takes a darker turn, evolving from an episodic torment into a relentless, chronic condition. Chronic migraine, defined as 15 or more headache days per month for at least three months, with at least eight of those being migrainous, represents a tragic transformation of the brain’s pain system.
In chronic headache, the process of central sensitization becomes ingrained. The pain pathways are no longer merely hypersensitive; they are fundamentally rewired. The TNC and higher cortical centers develop a persistent state of hyperexcitability, almost "learning" to be in pain. The descending pain modulatory systems may become dysfunctional, losing their ability to suppress pain effectively. This chronification often involves structural and functional changes in various brain regions, including reduced gray matter volume in pain-processing areas and altered connectivity within the pain matrix. The brain adapts to its painful state, making it incredibly difficult to break the cycle.
And what initiates this susceptibility in the first place? The answer often lies in our genetic code. While no single "headache gene" has been identified for common forms of migraine, strong genetic predispositions exist. Familial hemiplegic migraine, a rare form, has been linked to mutations in genes coding for ion channels (CACNA1A, SCN1A) and ATPases (ATP1A2), critical for neuronal excitability. More common migraines are polygenic, meaning multiple genes contribute to susceptibility, often interacting with environmental triggers. These genetic variations can influence:
- Neuronal excitability: Making certain brains more prone to CSD or general hyperexcitability.
- Neurotransmitter systems: Affecting the balance of serotonin, dopamine, CGRP, and other key players.
- Inflammatory responses: Influencing how the brain reacts to stress or injury.
- Blood vessel regulation: Modulating the reactivity of cerebral vasculature.
The story of the headache, therefore, is also a story of inheritance, a whispered legacy of vulnerability passed down through generations, waiting for the right confluence of internal and external factors to ignite the internal storm.
Conclusion: A Symphony Reimagined
The ache in the head, once dismissed as a simple discomfort, reveals itself as a marvelously intricate and terrifyingly complex neurobiological event. It is a story of whispers and roars, of delicate balances shattered, of ancient brain structures collaborating in chaos, and of the profound suffering that arises when the brain’s finely tuned symphony goes catastrophically off-key.
From the prodromal stirrings of the hypothalamus to the sweeping wave of cortical spreading depression; from the neurogenic inflammation unleashed by CGRP in the meninges to the relentless ascent of pain signals through the trigeminal system to the TNC; from the overwhelming of the thalamus to the widespread activation of the cortical pain matrix; and finally, to the insidious central sensitization and the lingering exhaustion of the postdrome – every stage is a chapter in this epic of internal turmoil.
Understanding this intricate narrative is not merely an academic exercise. It transforms our perception of pain, fostering empathy for those who live under its shadow and driving the relentless pursuit of more effective treatments. The emergence of CGRP-targeted therapies, for instance, represents a direct application of this scientific storytelling, interrupting a critical chapter in the headache’s progression.
The brain, in its infinite complexity, remains a frontier. But by dissecting the science of the ache, we gain not just knowledge, but a deeper appreciation for the intricate dance of neurons and chemicals, the profound vulnerability of our most vital organ, and the enduring human spirit that seeks to find solace and understanding even in the throes of its most intimate betrayal. The headache is not just a pain; it is a profound testament to the delicate, powerful, and sometimes tragic, opera of the mind.